Trethera Successfully Completes Phase 1 Dose Escalation Trial Demonstrating Broad Therapeutic Window, Favorable Safety, and Confirmed Target Engagement
News provided by
Oct 28, 2025, 9:57 AM ET
LOS ANGELES, Oct. 28, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, today announced successful completion of the dose-escalation portion of its ongoing Phase 1 solid tumor trial. The independent Safety Review Committee unanimously concluded that no dose-limiting toxicities occurred at any dose level, including the final and highest tested dose of 1,440 mg per day.
Patients tolerated daily doses of TRE-515 ranging from an initial 40 mg to a final 1,440 mg — a 36-fold increase — establishing the maximum tested dose and completing the trial safety portion. Antitumor activity and biomarker response were observed across multiple dose levels, indicating full target engagement and supporting a recommended Phase 2 dose well below the maximum tested dose.
TRE-515 inhibits deoxycytidine kinase (dCK), the key enzyme for the deoxyribonucleoside salvage pathway that becomes activated and essential for the growth of abnormal cells in autoimmune diseases and cancer. Blocking this pathway deprives cancer cells of the DNA building blocks needed for uncontrolled proliferation. Because dCK is relatively conserved in the regulated cell division of healthy cells, inhibiting dCK with TRE-515 has demonstrated favorable safety and potential clinical benefit in the ongoing first-in-human trial.
![Specialized [18F]CFA PET radiotracer of a patient with an extensive solid tumor, measuring dCK activity, both at baseline and after 28 days of treatment at 480 mg/day shows complete target engagement. The dark area in the right image reflects radiotracer excretion via the liver.](https://ml.globenewswire.com/Resource/Download/9d608da4-d848-445c-bac1-7285d6710ea1/picture1.png)
Figure 1: Specialized [18F]CFA PET radiotracer of a patient with an extensive solid tumor, measuring dCK activity, both at baseline and after 28 days of treatment at 480 mg/day shows complete target engagement. The dark area in the right image reflects radiotracer excretion via the liver.
The primary endpoints of the trial include safety and tolerability to define the maximum tolerated dose. Secondary endpoints assess pharmacokinetics, pharmacodynamics, antitumor activity, and biomarker-based evidence of target engagement. The trial incorporates two minimally invasive biomarkers: (i) the PET radiotracer [18F]CFA, which visualizes dCK activity, and (ii) plasma deoxycytidine (dC) levels, an indicator of dCK inhibition. The trial remains open, with multiple patients still on therapy.
Key results:
- Safety: TRE-515 was well tolerated with no observed dose-limiting clinical or laboratory toxicities. Most adverse events were mild (grade 1 or 2), transient, and manageable. Several patients received treatment for over 100 days with sustained tolerability.
- Dose: TRE-515 showed near-proportional exposure across doses, with rapid absorption (Tmax within 2 hours) and a plasma half-life exceeding 6 hours. Exposure levels were favorable relative to potency, even at 40 mg, the lowest dose tested.
- Antitumor Activity: Disease control occurred in 14 of the 30 patients (47%), including two low dose cohorts (40 mg and 80 mg). Durability lasted up to 342 days, despite patients being heavily pretreated (5th line therapy or later) with extensive metastases.
- Engagement Biomarkers: Imaging with the [18F]CFA PET probe confirmed complete target inhibition in multiple patients while on therapy (Figure 1). Plasma dC changes, the substrate for the enzyme dCK, provided compelling evidence (p <0.0001) of effective target engagement.
Earlier this year, the U.S. Food and Drug Administration (FDA) granted TRE-515 Fast Track designation in combination with radiation therapy for prostate cancer. The FDA Fast Track program is designed to accelerate the development of promising drug candidates targeting serious diseases with high unmet medical needs. With the maximum tested dose established, planning is now underway to (i) establish the recommended Phase 2 dose and (ii) explore combination therapy trials in prostate cancer.
“Establishing a therapeutic window reaches a pivotal milestone for the TRE-515 program,” said Dr. Ken Schultz, Trethera’s Chief Executive Officer and Chief Medical Officer. “These positive safety, target engagement, and biological activity results position TRE-515 as a promising new therapy for patients with solid tumors and autoimmune diseases."
“The biomarker data provide compelling evidence that TRE-515 achieves on-target inhibition of deoxycytidine kinase, validating its proposed mechanism of action,” said Dr. Michael Shepard, Lasker Laureate and Trethera Scientific Advisory Board member. “This precision targeting may prove pivotal in tumor growth suppression and combining with other therapies.”
Dr. Tim Donahue, Trethera Board Director and Chief of Surgical Oncology at UCLA, commented, "These encouraging results underscore the potential of TRE-515 to redefine treatment options for patients with advanced solid tumors. Targeting the salvage pathway explores an entirely new avenue of therapy—and one that could make a real difference for patients who have few remaining options.”
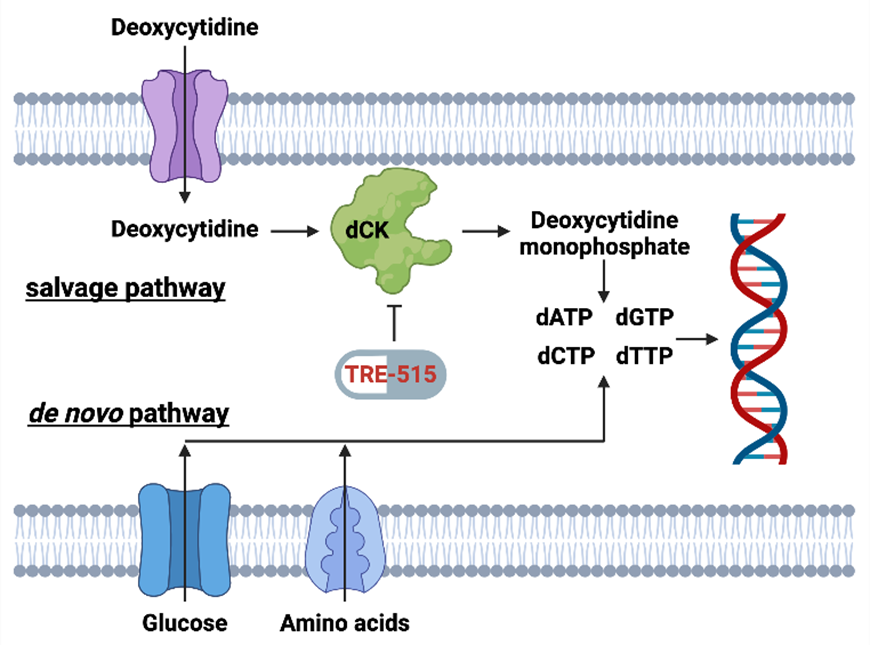
Figure 2: Biochemical pathways for the supply of deoxyribonucleoside triphosphate (dATP, dGTP, dCTP, and dTTP) pools. The salvage pathway becomes upregulated during autoimmune diseases and cancer. TRE-515 blocks the enzyme deoxycytidine kinase (dCK) in the deoxyribonucleoside salvage pathway.
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleoside metabolism led to the development of TRE-515, an orally administered capsule. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the deoxyribonucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. The FDA has designated TRE-515 a Fast Track drug for prostate cancer and an Orphan Drug for two autoimmune neurologic diseases. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/9d608da4-d848-445c-bac1-7285d6710ea1
https://www.globenewswire.com/NewsRoom/AttachmentNg/39a5f342-17ea-46f6-bc8a-7def6d014e7f

NOTE: This content is not written by or endorsed by "KTLA", its advertisers, or Nexstar Media Inc.
